Research Project
The last formation: dating recrystallisation events of evaporites using lyoluminescence
What are we up to?
We are supporting a sustainable future! Understanding Earth’s past 2.58 million years is crucial for mitigating climate change-induced risks to our modern societies. Climate and landscape change forecast models draw their data from studies about the past. The Marie Skłodowska-Curie project, lyoluminescence (LL), explores innovative ways to date past landscape changes. We aim to achieve a breakthrough in developing lyoluminescence as a dating tool on salts (sodium chloride and potassium chloride) for application in Earth Sciences.
We think that LL, naturally observable in salt minerals, says something about the last recrystallization event. The underlying idea is an old story, and various scholars have suggested the potential of halite as a material for optical luminescence dating, e.g., Bailey et al., (2000); Zhang et al., (2005). However, LL may offer an additional luminescence-dating tool for routine use in geochronology by targeting the crystallization instead of heat or light exposure events. If subsurface processes where hydration matters are of interest, LL may open new ways of understanding those processes.
How does it work? (the nutshell)
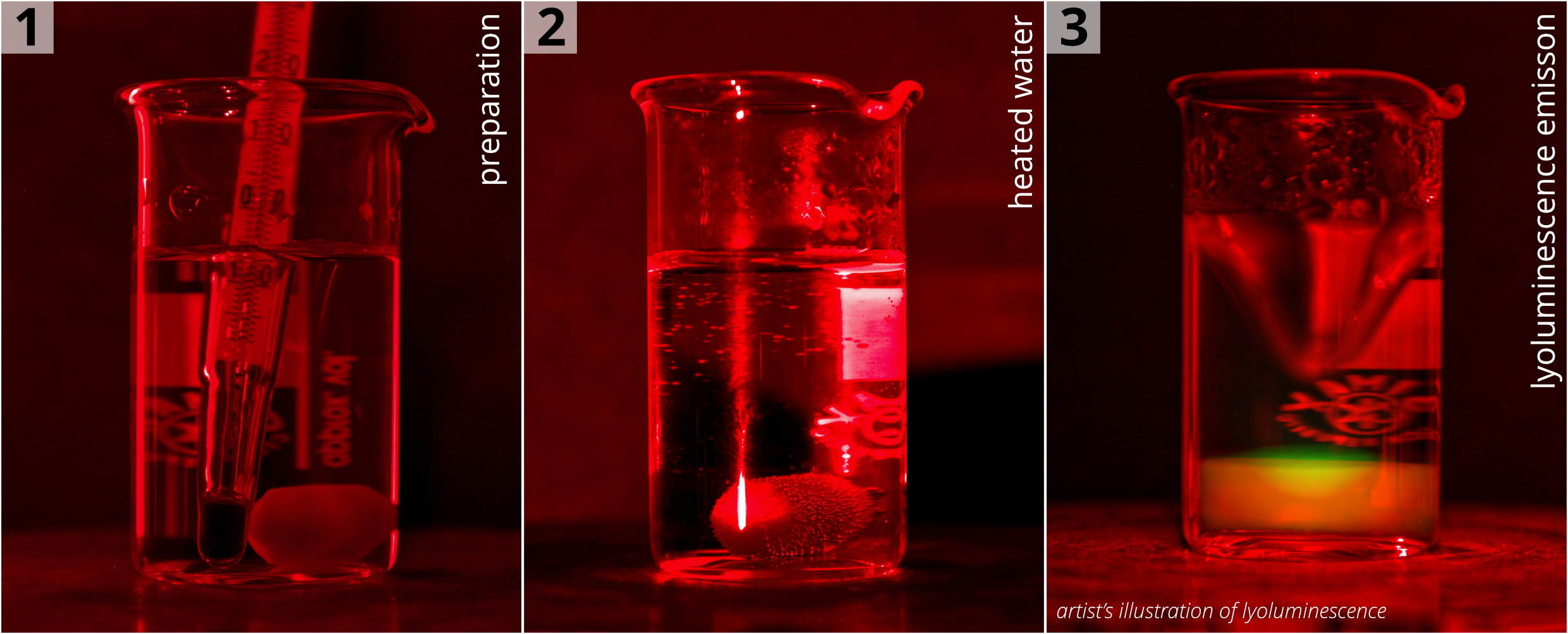
Lyoluminescence is a form of chemiluminescence wherein luminescence light is emitted during the solvation of previously irradiated crystals. An example of an LL signal observation experiment is depicted in Fig. 1. Here, sodium chloride previously exposed to ionizing radiation was dissolved in pure water at approximately 60 °C with the aid of magnetic stirring. However, it's important to note that the green glow seen in the right photo is merely an illustration of potential LL emission. Detecting such faint signals requires the use of highly sensitive devices, like a photomultiplier in single photon counting mode. Moreover, such measurements need to be conducted within a meticulously designed measurement chamber to prevent interference from external light sources.
Figure 1: Stages of conducting an LL experiment: 1) A beaker containing water and a stirring magnet positioned atop a magnetic stirrer; 2) Heating the water to a specific temperature; 3) Introducing previously irradiated salt (not depicted in the photo) into the beaker while stirring to observe the LL phenomenon. The green glow was added during the post-processing of the photo to illustrate the lyoluminescence signal; in our case, the sensor of the commercial camera used to take the photo lacked the necessary sensitivity to detect the faint LL signal.
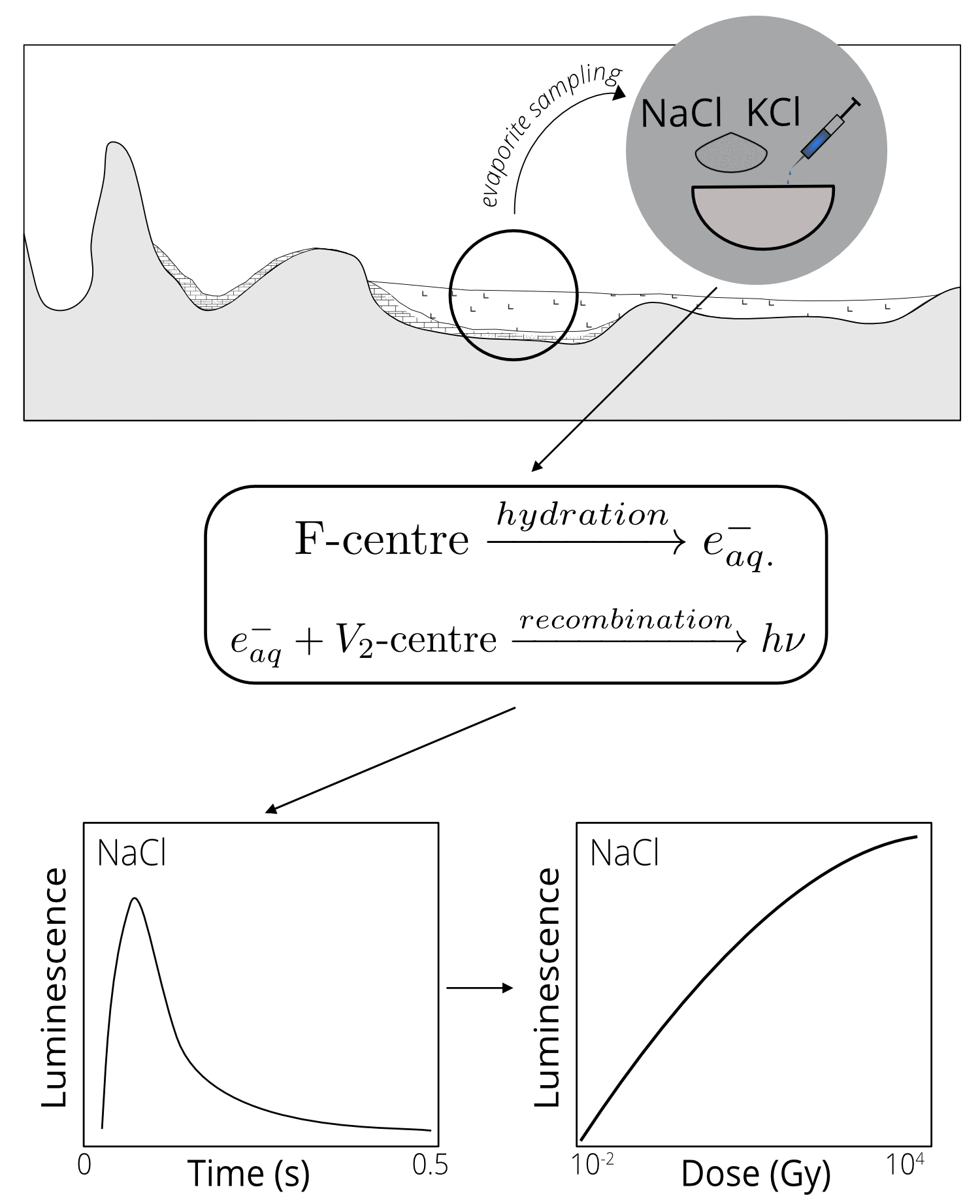
This phenomenon was initially described by Wiedemann and Schmidt in 1895. According to the current, simplified understanding of LL mechanisms (see Fig. 2) proposed by Atari in 1980, when an alkali halide crystal comes into contact with a solvent (such as water), it releases a hydrated electron (e-aq) from an F-center (the defect that captured the electron). Subsequently, the rapid recombination of the hydrated electron, primarily with a V2-center, at the water-solid interface results in light emission, known as lyoluminescence (in Fig. 2 depicted as hν). The typical shape of the LL signal (referred to as the LL glow curve) recorded with a photomultiplier tube in time is illustrated in Fig. 2, depicted in the graph on the left. The intensity of LL light increases proportionally with the radiation dose absorbed by the material after crystallization (see Fig. 2 graph on the right). This positive correlation between radiation dose and LL light emission makes crystal lattice defects suitable as natural ionizing radiation dosimeters. For instance, sodium chloride dissolved in pure water exhibits a saturation dose of approximately 10 kGy (Atari et al., 1973). Given realistic dose rates of about 4 Gy/ka (Han et al., 2014), the LL signal from sodium chloride minerals could potentially determine an age of up to 2.5 million years!
Figure 2: The concept of lyoluminescence.
Where can it be applied?
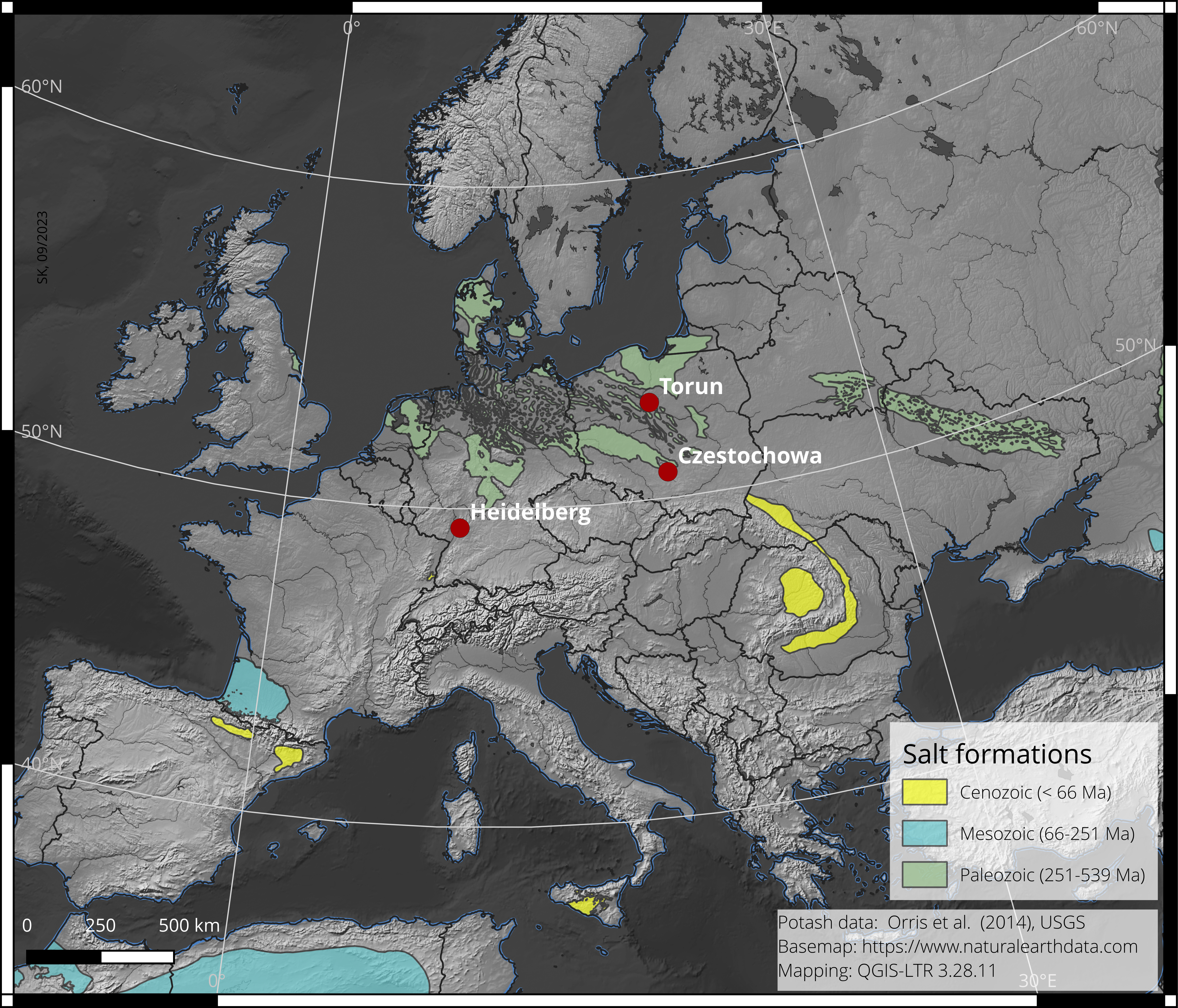
Widely distributed natural deposits (as depicted in Fig. 3), highly soluble in water, and salts such as halite and/or sylvite found in areas of high aridity are promising candidates for LL dating applications. Of particular interest are naturally coloured alkali halides, including yellow to brownish varieties and the blue variant of rock salt (Atari et al., 1973), which are characterized by a significant concentration of radiation-induced crystal defects (an example of blue halite is shown in Fig. 4).
Figure 3. Locations of known large salt deposits in Europe.
Figure 4. Photo of blue halite – Geological Museum of the State Geological Institute in Warsaw, Poland (taken by Magdalena Biernacka)
What are the project objectives?
- Pooling strategic knowledge on lyoluminescence on evaporites and its potential applications, specifically halite and sylvite.
- Design, develop and test a new portable lyoluminescence reader.
- Apply the newly developed device to natural salt and develop dating protocols and analysis routines; if successful, map out a commercialization plan.
References
- Atari, N.A., 1980. Lyoluminescence mechanism of gamma and additively coloured alkali halides in pure water. Journal of Luminescence 21, 305–316. https://doi.org/10.1016/0022-2313(80)90009-5
- Atari, N.A., Ettinger, K.V., Fremlin, J.H., 1973. Lyoluminescence as a possible basis of radiation dosimetry. Radiation Effects 17, 45–48. https://doi.org/10.1080/00337577308232596
- Bailey, R.M., Adamiec, G., Rhodes, E.J., 2000. OSL properties of NaCl relative to dating and dosimetry. Radiation Measurements 32, 717–723. https://doi.org/10.1016/S1350-4487(00)00087-1
- Han, W., Ma, Z., Lai, Z., Appel, E., Fang, X., Yu, L., 2014. Wind erosion on the north‐eastern Tibetan Plateau: constraints from OSL and U‐Th dating of playa salt crust in the Qaidam Basin. Earth Surf Processes Landf 39, 779–789. https://doi.org/10.1002/esp.3483
- Wiedemann, E., Schmidt, G.C., 1895. Ueber Luminescenz von festen Körpern und festen Lösungen. Annalen der Physik und Chemie 292, 201–254. https://doi.org/10.1002/andp.18952921004
- Zhang, J.F., Yan, C., Zhou, L.P., 2005. Feasibility of optical dating using halite. Journal of Luminescence 114, 234–240. https://doi.org/10.1016/j.jlumin.2005.01.009
Milestones
-
What did we do so far?
- The first portable LL reader prototype was completed In progress:
- First LL signal from natural samples
- New dating protocol for LL
- Commercialisation strategy for LL equipment